You can contact LEARNZ, part of CORE Education, at:
Postal Address:
PO Box 13 678,
Christchurch 8141,
New Zealand
Water can change from a liquid to a solid or a gas and back to a liquid, over and over again. Adding or removing heat can change the state water is in.
Water, like all other types of matter, needs energy to be added or removed to change states.
A block of ice is solid water. When heat (a form of energy) is added, the ice melts into liquid water. It has reached its melting point at 0°C. If you were to keep heating it, then the water will turn into water vapour (gas). The water has reached its boiling point at 100°C.
If heat is removed from water vapour, the gas cools down and it condenses back into liquid water. Keep cooling the water (by removing heat), and it becomes solid ice. This is its freezing point.
Pressure is the force (for example your weight) applied to the surface of an object (for example the floor). When you stand up you apply pressure to the floor.
Air pressure
Air pressure is the weight of the air pushing down on the earth. At sea level this pressure is called 1 Atmosphere. The air pressure at the top of Mt Everest is half of this (0.5 Atmosphere).
If you were to boil water high up in the mountains, thousands of metres above sea level, you would not need to heat it as much because there is less air pressure. The air pressure is lower because there is less weight of air above – the air is not as dense.
Less pressure means it is easier for the molecules to move and less heat needs to be added to cause a change in state.
Watch this video to see different temperatures for boiling water at different altitudes.
Water pressure
Water pressure is the weight of the ocean pushing down on you. The deeper you go under the sea, the greater the pressure of the water pushing down on you. For every 10 meters you go down, the pressure increases by 1 Atmosphere. In the deepest ocean, the pressure is over 1,000 atmospheres, or the same as the weight of an elephant balanced on a postage stamp.
Water under pressure is harder to freeze! Water below freezing point can still be liquid under pressure! Watch this video where a bottle of cola put in the freezer only turns to ice when the cap is removed releasing the pressure.
This is very important in Antarctica where very deep water may be below freezing temperature. It is called supercooled water. Supercooled water is water that is still liquid even though it is below the freezing point.
The oceans are salty so they don’t freeze at the same temperature as fresh water. Salt lowers the freezing point so sea water freezes at about -2 degrees Celsius. This is why salt is used on some roads in winter to stop ice from forming.

,Puddles of water evaporate into water vapour as they heat up. Heat allows water molecules to move faster until they are able to break away from each other and escape as a gas. Image: LEARNZ.

,Ice usually forms below 0 degrees Celsius. This is known as its freezing point. What can change the freezing point of ice? Image: LEARNZ.
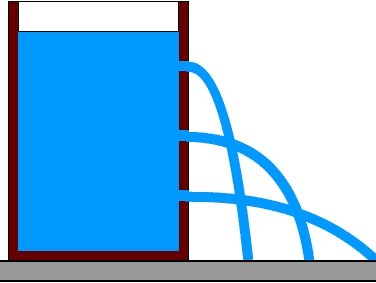
,This is a demonstration of water pressure: pressure near the surface is less than the pressure deeper down. Image: Public Domain.

Antarctica is known as the frozen continent. How do you think a small increase in average temperature could affect Antarctica? Image: LEARNZ.
Can you find out more about supercooling and when it happens?